The Long Road to mRNA Vaccines: From Early Discoveries to a Pandemic Breakthrough
Introduction
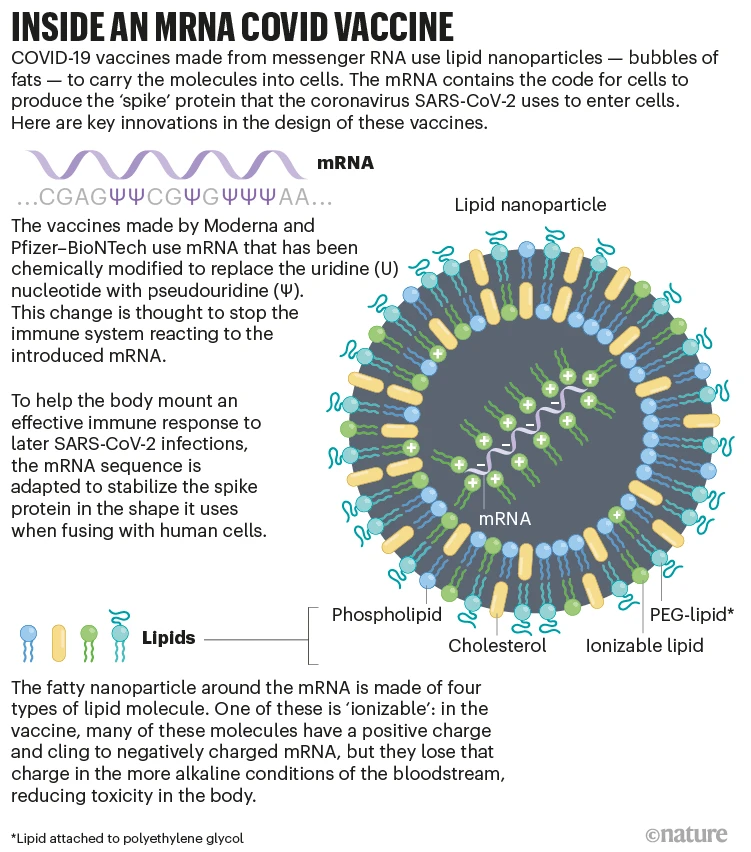
It was such an excitement to read the history review of the tangled history of mRNA vaccines by Elie Dolgin. Understanding the steps towards success is very helpful for us to appreciate the importance of each component in mRNA-LNP. And also why Karikó and Weissman merit the Nobel prize.
The Early History of mRNA Research
In the 1970s, mRNA was first discovered, allowing scientists to explore its potential. By 1978, they had succeeded in using liposomes to deliver lab-made mRNA into mouse and human cells to induce protein production. Over the next decade, key advances were made in synthesizing mRNA in the laboratory and delivering it enclosed in positively-charged liposomes to enhance cellular uptake. In 1989, Robert Malone performed a landmark experiment showing frog embryo cells could take up lipid-encapsulated mRNA. This sparked recognition of mRNA’s promise as a new drug platform. However, research remained focused on studying basic molecular biology, rather than developing mRNA medicines.
Through the 1980s-1990s, scientists continued investigating mRNA mainly as an experimental tool to unravel genetic and cellular processes. Use of mRNA therapeutics was still limited by instability of lab-synthesized strands and difficulty manufacturing pure, non-immunogenic batches. The predominant view was that mRNA technology was too premature to yield viable drugs or vaccines in the near future.
Barriers to mRNA Vaccine Development
A few pioneering researchers pressed on with mRNA vaccine experiments in the 1990s, though progress remained slow. Biotech startups like CureVac and Argos Therapeutics began testing unmodified mRNA treatments, but encountered problems with inflammatory immune reactions hampering results. Such biotech firms struggled to attract investors to fund optimization and commercialization. At the same time, major pharmaceutical companies like Merck and Roche initiated and then abandoned mRNA vaccine programs due to perceived manufacturing barriers and inadequate investment incentives.
Without solutions to instability and immunogenicity problems, most companies judged mRNA vaccines too risky and difficult to synthesize at scale. The majority of industry research dollars flowed into alternative technologies like DNA vaccines and viral vectors. Scientists urging focus on tackling mRNA vaccine challenges faced scepticism and rejection rather than support. Despite nascent promise, mRNA vaccines languished with little funding or corporate development through the 1990s-2000s.
Solving the Immune Response Problem
A breakthrough came when Katalin Karikó and Drew Weissman demonstrated that substituting true uridine with modified pseudouridine nucleotides prevented inflammatory activation of immune sensors by lab-made mRNA. This discovery finally provided a path to make mRNA minimally immunogenic by evading innate immune defenses. Before this, generating high-protein yields from delivered mRNA was obstructed by the body destroying injected mRNA before useful translation could occur.
Karikó and Weissman published their pivotal findings in 2005 after years devoted to solving issues around mRNA’s immunogenicity. Their perseverance laid the foundation to transcribe modified mRNA that appeared “normal” to the body, overcoming a huge roadblock. Following this work, in 2010, Derrick Rossi’s successful demonstration of using modified mRNA to transform cell fates gained substantial attention. The technology no longer seemed so far-fetched, spurring modern pioneers like BioNTech and Moderna to tackle therapeutic mRNA.
Enabling Delivery Through Lipid Nanoparticles
Another central challenge holding mRNA vaccines back was finding an optimal delivery system to safely encapsulate the mRNA and carry it into human cells. Biochemist Pieter Cullis leveraged progress with lipid nanoparticles (LNPs) for gene therapy to develop modified LNPs for mRNA. His custom lipid cocktail used ionizable cationic lipids that bound and protected mRNA during delivery, while avoiding toxicity.
Cullis helped advance LNP siRNA treatments to human trials throughout the 2000s. Around 2012, based on this foundation, LNP formulations were tailored for mRNA cargoes. Effectively formulated, scalable LNP production methods were devised leveraging microfluidic systems. These lipid carriers overcame mRNA’s fragility and enabled transit to cytosol translation sites. By shielding and transferring mRNA with minimized immune detection, LNPs finally facilitated clinical testing of Moderna’s first LNP mRNA vaccine in 2015.
mRNA Vaccines’ Pandemic Success
After over 40 years of incremental advances, the COVID-19 pandemic ignited an mRNA vaccine breakthrough. Swift collaboration between Vision and BioNTech, followed by Moderna, marshalled substantial resources to rapidly design, manufacture, and test mRNA vaccines against SARS-CoV-2. Owing to prior groundwork optimizing critical mRNA modifications and LNP delivery systems, the vaccines progressed from concept to authorization in a record timeframe.
The exceptional clinical trial results demonstrated both the technology’s safety and outsized 95% efficacy against COVID-19 disease. This dramatic demonstration of real-world performance finally overcame mainstream reservations that stalled development for decades. Their overwhelming success boosted mRNA vaccine prominence almost overnight. Now poised to disrupt everything from influenza to cancer applications, investors and big pharma are racing to grab a piece of the estimated $50B market through partnerships with pioneers like Moderna and BioNTech.
Off note:
It was great to know that the RNAi and mRNA vaccines somehow was originated from the same place, as delivering mRNA into cells not only induces mRNA expression but also induces gene silences.
Order option:
ALC0135, N1-Me-Pseudo UTP Trisodium solution