
As a close observer on the mRNA-LNP field, the recent developments in mRNA design and optimization reported by Professor Xiao Wang’s team at MIT Perticularly catching my eye. Their work, published in Nature Biotechnology, represents a significant leap forward in our understanding and manipulation of mRNA structures for therapeutic applications.
Innovative mRNA Assembly Strategy
The team’s RNA LEGO approach is particularly exciting. This modular assembly strategy allows for unprecedented control over mRNA structure, enabling the exploration of chemical modifications and topological restructuring at both 5′ and 3′ ends. This level of precision in mRNA engineering has been a long-standing goal in the field, and it’s gratifying to see such progress.
Multi-capped mRNA: A Game-Changer?
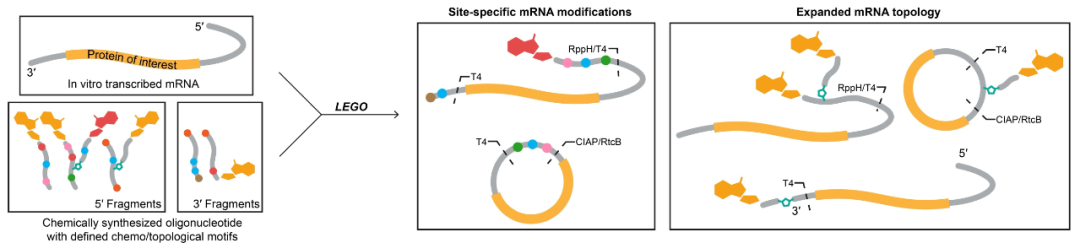
The concept of multi-capped mRNA is intriguing. By introducing multiple cap structures at the 5′ end, the researchers have potentially found a way to accelerate the formation of translation initiation complexes. The reported 20-fold increase in peak protein expression is remarkable and could have far-reaching implications for mRNA therapeutics.
However, we must approach these results with cautious optimism. While the in vitro and mouse model results are promising, it remains to be seen how these modifications will perform in human clinical trials. The complexity of the human immune system and potential off-target effects need to be thoroughly investigated.
Circular RNA with Cap Structures
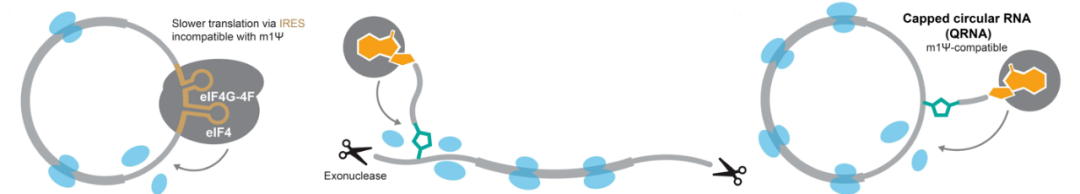
The development of capped circular RNA, or “QRNA,” is another fascinating advancement. Circular RNAs have always been attractive due to their stability, but their low translation efficiency has been a significant hurdle. The ability to introduce cap structures to circular RNA could potentially combine the best of both worlds – stability and efficient translation.
Mechanistic Insights
The proposed “cap proximity-driven translation” model provides valuable insights into mRNA translation mechanisms. This model could open up new avenues for mRNA design and potentially explain some previously observed phenomena in natural mRNA regulation.
Critical Considerations
- Scalability: The complex synthesis methods described may pose challenges for large-scale production necessary for widespread therapeutic use.
- Immunogenicity: Highly modified mRNAs might trigger unexpected immune responses. Extensive safety studies will be crucial.
- Cost: The intricate modifications could significantly increase production costs, potentially limiting accessibility to these advanced therapies.
- Long-term effects: The long-term consequences of introducing such heavily modified mRNAs into biological systems are unknown and will require careful monitoring.
Future Prospects
Despite these concerns, the potential applications in vaccines and protein replacement therapies are enormously promising. The reported improvements in SARS-CoV-2 vaccine efficacy and hEPO expression in mice are particularly encouraging.
As we move forward, it will be crucial to balance the excitement of these new possibilities with rigorous safety assessments and ethical considerations. The mRNA-LNP field is evolving rapidly, and this work by Wang’s team represents a significant milestone in our journey towards more effective and versatile mRNA therapeutics.